(Image source from: odishabytes.com)
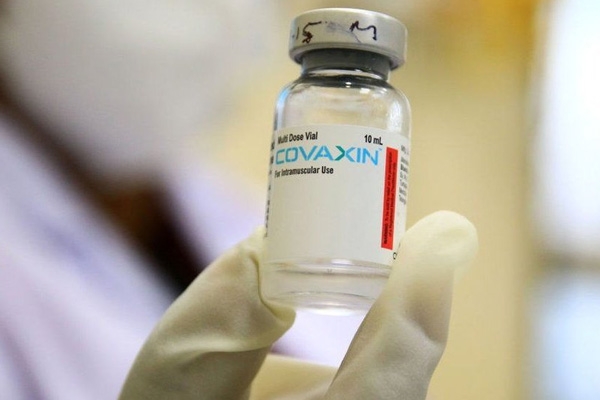
The expert panel from the Central Drug Authority approved the granting of emergency use of Covaxin of Bharat Biotech for children aged between 2 and 18 years with certain conditions. Bharat Biotech completed the second and third phase of trials of Covaxin for children. The data has been submitted to the Central Drugs Standard Control Organization (CDSCO) for its verification and emergency use authorization (EUA). The Subject Expert Committee (SEC) examined the data and approved the emergency use of Covaxin for children.
After detailed deliberation, the committee recommended grant of market authorization of the vaccine for the age group of 2 to 18 years for restricted use in emergency situations subject to the certain conditions," told the statement. The recommendations are forwarded to the Drug Controller General of India for the final approval. The vaccination for children will be launched at the earliest and will be available in the market. The final approval will be given by the Drug Controller General of India soon. In August, Zydus Cadila's three-dose DNA jab was allowed for children over 12 years and Covaxin is the second vaccine cleared for usage in India. Serum Institute's Novavax is the other vaccine for children that cleared the trials. Biological E's Corbevax is the fourth vaccine that cleared the trials for children above the age of five years.
The efficacy rate is the same for children as that of adults for Covaxin and the vaccine is said to be 77.8 percent effective. For now, India has vaccinated 30 crores of its 130 crore population fully.
By Siva Kumar