Bharat Biotech Starts First Phase Human Trials of ‘COVAXIN’ at Rohtak in Haryana
July 18, 2020 16:37
(Image source from: assettype.com)
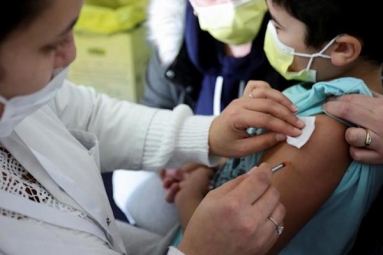
First phase human trial of Bharat Biotech’s anti-COVID-19 vaccine ‘Covaxin’ has been started on July 15 in Rohtak.
Haryana Health Minister, Anil Vij in a tweet said that human trials on Covaxin have been started at PGI Rohtak and has 375 volunteers for this first phase of testing.
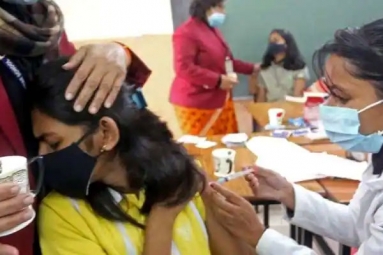
On July 15, the vaccine was injected into three people and according to the health minister of Haryana, all three of them responded well to the vaccine and there were no adverse effects.
Bharat Biotech got the license for human trials of its COVID-19 vaccine from DGCI recently. There are over 7 coronavirus vaccines at various stages in the country which are ready for human trials and 2 of them have already received the approval from DGCI. Bharat Biotech is the pioneer to get the permission.
The second company is Zydus and it has also recently received approval for human trials of its vaccine for coronavirus.
The Hyderabad based company Bharat Biotech has jointly developed Covaxin with Indian Council od Medical Research (ICMR). As per a report, the first phase of human trials from Bharat Biotech will be conducted on 375 volunteers.
12 hospitals in different parts of the country will carry out the first phase human trials of which PGI Rohtak is the first location.
The vaccine manufacturer had said that these are randomised double blind clinical trials and some of the people who have volunteered for this will be vaccinated and some of them will be given a placebo.
However, neither the manufacturer nor the volunteer would know who was injected with the vaccine. They will only come to know after the test period is over.
The first phase of the trials is all about testing the volunteers for any adverse effects being developed after the vaccine is injected.
Whether the vaccine works against the coronavirus disease will be known in the second stage of human trials of Covaxin.
ICMR had written to 12 institutes across the country where the first phase of trials to be conducted, to ensure all the necessary approvals are received from the internal committees and the volunteers are gathered for the purpose by July 7.
Covaxin has been derived from the strain of a novel coronavirus isolated by the National Institute of Virology in Pune.
Once the vaccine is injected into humans, it has no potential to infect as it is a killed virus. It goes into the immune system of the person and creates an antibody response towards the virus.
By Gayatri Yellayi